


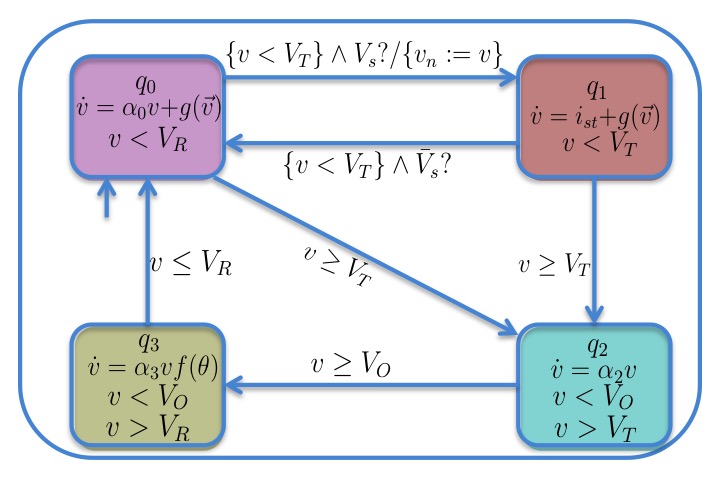
 This model (adapted from Ye et al. CMSB 2005)
describes the electrical conduction system (ECS) of the heart as a network of cardiac
cells. Cells are connected by pathways through which the action potential (AP) is propagated.
Propagation delays depend on the physiology of the tissue considered, and can be
tuned to reproduce various diseases. Each cell is a hybrid automaton describing the main phases of
cardiac AP:
This model (adapted from Ye et al. CMSB 2005)
describes the electrical conduction system (ECS) of the heart as a network of cardiac
cells. Cells are connected by pathways through which the action potential (AP) is propagated.
Propagation delays depend on the physiology of the tissue considered, and can be
tuned to reproduce various diseases. Each cell is a hybrid automaton describing the main phases of
cardiac AP:
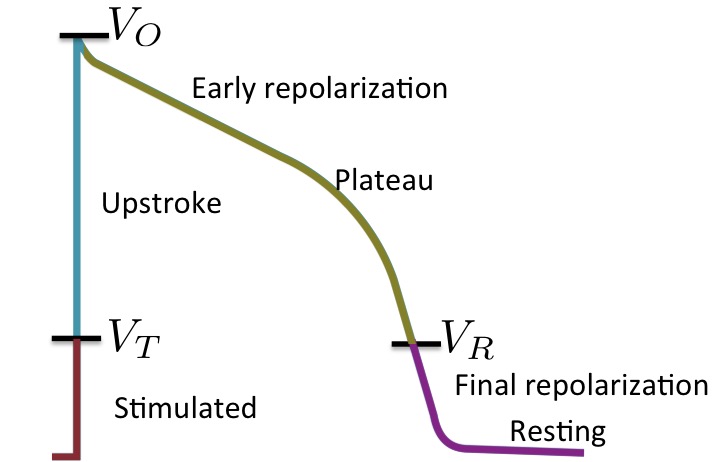
resting and final repolarisation (mode q0 in the figure),
stimulated (mode q1), upstroke (q2), and plateau and early repolarisation (q3).
A MATLAB Simulink version of the model with 34 cells can be downloaded through the link below.
Download model
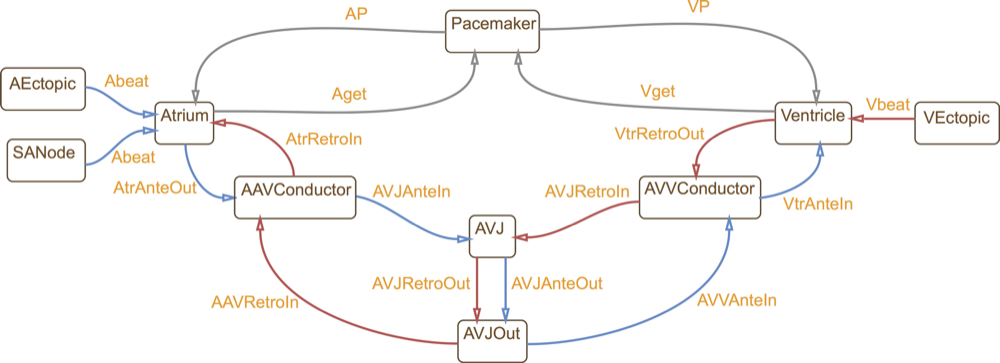 This is a hybrid automata translation of the IDHP (Integrated Dual-chamber Heart and Pacer) model by
Lian et al.
This model consists of 9 conduction nodes and two main conduction pathways: the antegrade conduction,
that is, the normal situation where an AP generated by the sinoatrial (SA) node
stimulates the atria and is conducted to the ventricles through the atrio-ventricular (AV) node;
and the retrograde conduction, where the impulse travels in the opposite direction
(from the ventricles to the atria through the AV node). Retrograde conduction occurs with artificial pacing
or ectopic beats. Each conduction node is a hybrid automaton component and conduction between nodes is implemented through
synchronisation between the involved components. In this way, the model can be easily extended with
other accessory conduction pathways in order to reproduce specific heart conditions.
This is a hybrid automata translation of the IDHP (Integrated Dual-chamber Heart and Pacer) model by
Lian et al.
This model consists of 9 conduction nodes and two main conduction pathways: the antegrade conduction,
that is, the normal situation where an AP generated by the sinoatrial (SA) node
stimulates the atria and is conducted to the ventricles through the atrio-ventricular (AV) node;
and the retrograde conduction, where the impulse travels in the opposite direction
(from the ventricles to the atria through the AV node). Retrograde conduction occurs with artificial pacing
or ectopic beats. Each conduction node is a hybrid automaton component and conduction between nodes is implemented through
synchronisation between the involved components. In this way, the model can be easily extended with
other accessory conduction pathways in order to reproduce specific heart conditions.
A MATLAB Stateflow version of the model can be downloaded through the link below. The archive includes the heart model and its composition with the dual-chamber pacemaker model of Jiang et al. and derived from the Boston Scientific open specification.
Download model
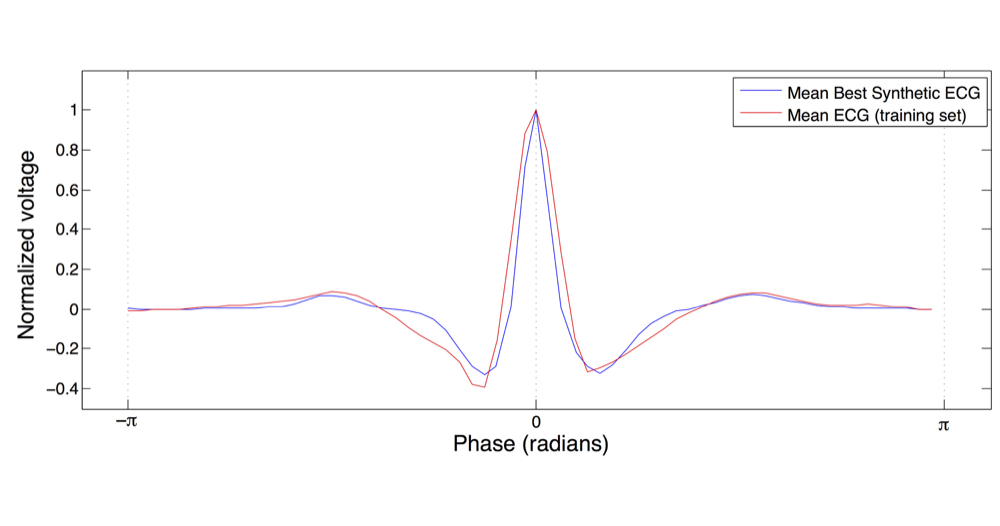 The IDHP model can be parametrised from patient's electrocardiogram (ECG) in order to reproduce
the specific physiological characteristics of the individual. For this purpose, we extended the IDHP
model in order to generate synthetic ECG signals that reflect the heart events occurring at simulation time.
Starting from raw ECG recordings, our method automatically finds model parameters such that the synthetic signal best mimics the
input ECG, by minimising the statistical distance between the two.
The IDHP model can be parametrised from patient's electrocardiogram (ECG) in order to reproduce
the specific physiological characteristics of the individual. For this purpose, we extended the IDHP
model in order to generate synthetic ECG signals that reflect the heart events occurring at simulation time.
Starting from raw ECG recordings, our method automatically finds model parameters such that the synthetic signal best mimics the
input ECG, by minimising the statistical distance between the two.
Model personalisation from ECG is available in the HeartVerify tool.
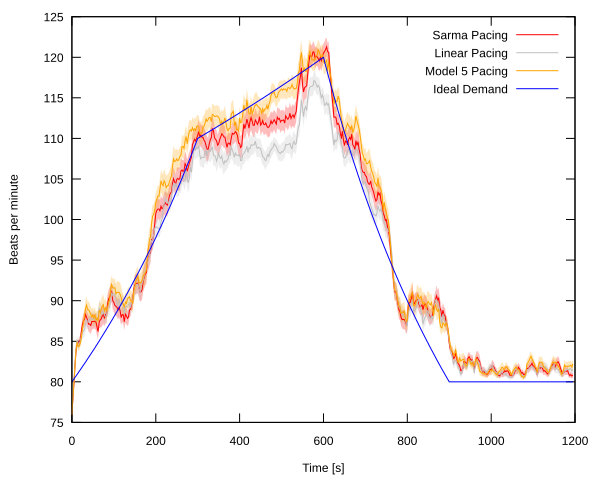 Rate-adaptive (RA) pacemakers are able to adjust the pacing rate depending on the levels of
activity (physical, mental or emotional) of the patient, detected by processing physiological signals data.
RA pacemakers represent the only choice for individuals
with chronotropic incompetence, that is, when the heart rate cannot naturally
adapt to increasing demand (e.g. AV block).
We developed a model of a RA pacemaker based on a QT interval (QTI) sensor,
a highly specific metabolic sensor that exploits the fact that physical activity
shortens the QT interval (a feature of the ECG), and thus requires an increased heart rate.
The RA pacemaker model can be used with both generic and personalised heart models to verify
properties such as: malfunctioning of physiological sensors that can lead to unsafe heart rhythms; or
adequacy of the rate-adaptation algorithm during typical exercise activity (see figure), i.e. its ability to provide a
pacing rate that reflects patient's metabolic demand.
Rate-adaptive (RA) pacemakers are able to adjust the pacing rate depending on the levels of
activity (physical, mental or emotional) of the patient, detected by processing physiological signals data.
RA pacemakers represent the only choice for individuals
with chronotropic incompetence, that is, when the heart rate cannot naturally
adapt to increasing demand (e.g. AV block).
We developed a model of a RA pacemaker based on a QT interval (QTI) sensor,
a highly specific metabolic sensor that exploits the fact that physical activity
shortens the QT interval (a feature of the ECG), and thus requires an increased heart rate.
The RA pacemaker model can be used with both generic and personalised heart models to verify
properties such as: malfunctioning of physiological sensors that can lead to unsafe heart rhythms; or
adequacy of the rate-adaptation algorithm during typical exercise activity (see figure), i.e. its ability to provide a
pacing rate that reflects patient's metabolic demand.
Our paper on rate-adaptive pacemakers won the best paper award at IEEE ICHI 2014 conference!
An implementation of the rate-adaptive model is available in the HeartVerify tool.